TESTING FOR TOXICITY,MUTAGENICITY & GENOTOXICITY
Testing for Toxicity
Both short-term or acute and long-term or chronic toxicity testing are carried out to determine the toxic effects of the drug and mortality in animal models.All these tests are carried out under a standard procedure of "GOOD LABORATORY PRACTICE" to safeguard the quality, integrity, and safety of the preclinical trials. Drug Development: Drugs must be approved by the FDA before they can be marketed in the US. Long, costly, and inherently risky: only 1 of 10-15,000 reach FDA approval. Toxicology testing is required to demonstrate that drugs are safe before they can be given to humans.
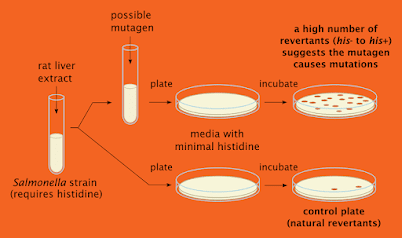
Testing of Mutagenicity:-
Mutagenicity:-
Mutagenicity refers to the induction of permanent transmissible changes in the amount or structure of the genetic material of cells or organisms. These changes may involve a single gene or gene segment , a block of genes or chromosomes.
Testings:-




Comments
Post a Comment